Introduction
Aflatoxin, a secondary metabolite synthesized by fungi such asAspergillus flavus and Aspergillus parasiticus via the polyketidepathway, typically comprises a difuran ring and an oxinone.Natural aflatoxins B1, B2, G1, and G2 are classified basedon their chemical structures. These mycotoxins, present incontaminated crops used for livestock feed, can accumulate inmeat, eggs, milk, and other foods, posing health risks throughthe food chain.
Biocomma established a high-throughput LC-MS/MS methodusing Copure® 226 Multifunctional Purification Plate fordetection of aflatoxins B1, B2, G1, and G2 in corn flour withgood recovery and stability for your reference. The recoverieswere 90-110%, and CV value between wells was less than5% at both 8 ng/g and 16 ng/g concentrations. This method isalso applicable to cereals, cereal products, nuts, seeds, oils,condiments, infant formulas, and supplementary foods.

Experiment
Extraction
Weigh 5 g of sample into a 50 mL centrifuge tube. Add isotopeinternal standard and vortex to mix well. Let stand for 30 min.Add 20 mL of acetonitrile-water solution (84:16) and vortexto mix well. Then, extract by ultrasonic treatment for 20 min.Centrifuge at 6000 r/min for 10 min. The supernatant is readyfor purification.
Purification (Copure® 226 Multifunctional PurificationPlate)
Place a 24 well purification plate on a 24 well collection plate.Add 6 mL of supernatant to the purification plate. Place the 24well purification plate and the collection plate on the Biocomma®Positive Pressure 24 Processor. Turn on the gas. Make surethe wells of purification plate are positioned directly underthe gas vent. Adjust the gas flow until all the sample filter intothe collection plate. Pipette 4 mL of sample to a clean tube.Evaporate sample to dryness at 40 ℃ and redissolve by 1 mLof initial mobile phase. Vortex for 30 seconds to dissolve theresidue. After filter by 0.22 μm microporous membrane, thesample is ready for analysis.
Instrument Conditions
1.Chromatography Conditions
Equipment: UPLC-MS/MS (Thermo Scientific TSQ Endura)
Chromatographic column: Thermo C18 (2.1 mm×100 mm, 1.7um)
Mobile Phase: A: Water (0.1% Formic acid), B: AcetonitrileMobile Phase Gradient: Initial 80%A, 20%A (0 min~2min), 20%A (2 min~3 min), 80%A (3 min~3.1min), 80%A(3.1min~5min)
Flow Rate: 0.3 mL/min
Column Temperature: room temperature
Injection Volume: 5.0 μL
2.Mass Spectrometry Conditions
Detection method: MRM
Table 1. Ion Source Control Conditions
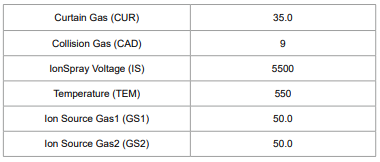
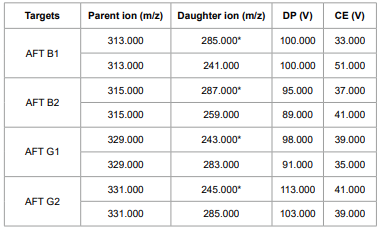
Table 2. Ion Selection Parameters (*Quantitative Ions)
Results

Figure 1. Decolorization Effect
① Before Purification
② Purified by Copure® 226 Multifunctional Purification Plate
Based on Figure 1, the pigments are obviously absorbed byCopure® 226 26 Multifunctional Purification Plate.
Table 3. Aflatoxin Spike in Corn Flour Recovery
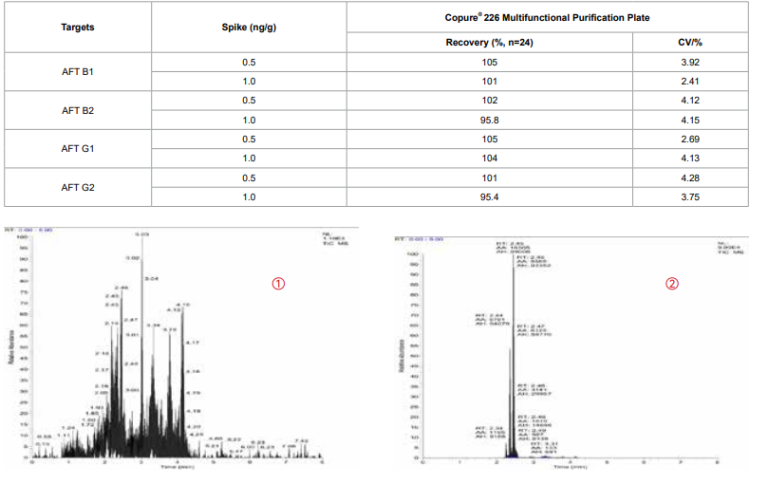
Figure 2. TIC of Corn Flour Sample
① Before Purification
② Purified by Copure® 226 Multifunctional Purification Plate
Based on Table 3 and Figure 2, the miscellaneous peaks of purified sample are much fewer indicating obviously absorbed impurities.The recovery rates of aflatoxin in the 24 wells are between 90-110%, and the CV value is less than 5%, which meet the standard ofexperimental requirements.